by Debra Heine
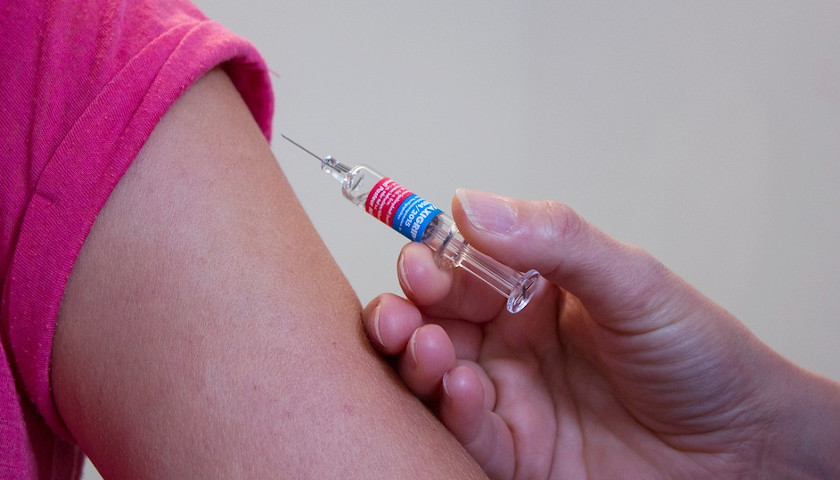
The FDA and Centers for Disease Control and Prevention announced Tuesday that they are recommending that the use of the single-dose Johnson & Johnson vaccine be halted after six cases of a rare and severe type of blood clot were reported in the United States.
The dangerous clots developed about two weeks after the vaccine was administered in these patients—all of them women between the ages of 18 and 48, according to ABC News.
“Safety is a top priority for the federal government,” acting FDA Commissioner Dr. Janet Woodcock told reporters in a virtual news briefing, adding that while the blood clots were “extremely rare” the government was acting “out of an abundance of caution.”
“We are committed to patient safety,” Woodcock said, while encouraging Americans to continue to get vaccinated with the experimental Pfizer and Moderna vaccines.
“[The] CDC will convene a meeting of the Advisory Committee on Immunization Practices (ACIP) on Wednesday to further review these cases and assess their potential significance,” read a joint CDC and FDA statement issued earlier Tuesday morning. “[The] FDA will review that analysis as it also investigates these cases.”
The recommended pause in J&J vaccinations is not expected to last long.
“The timeframe will be determined by what we learn in the next few days. However, we expect it to be a matter of days for this pause,” Woodcock said.
More than 6.8 million doses of the J&J vaccine have already been administered in the United States.
Several states as well as CVS and Walgreens announced they would stop administering the J&J vaccine immediately.
“This is a recommendation, not a mandate,” Dr. Peter Marks, the director of the FDA’s Center for Biologics Evaluation and Research, said on the briefing call, adding Americans should talk to their health care providers about their personal situation.
Marks said there’s no clear connection with women taking birth control, at this time, but women on the pill have been sharing their stories.
“I've been in the hospital for a week, and I was in the ICU until Sunday,” she said.
She said she had no pre-existing conditions, and no family history of clotting. “The only thing that is potentially correlated is I was on an estrogen-based birth control,” she shared.
— Amanda Golden (@amandawgolden) April 13, 2021
“In these cases, a type of blood clot called cerebral venous sinus thrombosis (CVST) was seen in combination with low levels of blood platelets (thrombocytopenia),” the statement read. “Treatment of this specific type of blood clot is different from the treatment that might typically be administered. Usually, an anticoagulant drug called heparin is used to treat blood clots. In this setting, administration of heparin may be dangerous, and alternative treatments need to be given.”
CVST occurs when a blood clot forms in the brain’s venous sinuses, preventing blood from draining out of the brain, according to John Hopkins Medicine.
As a result, blood cells may break and leak blood into the brain tissues, forming a hemorrhage.
This chain of events is part of a stroke that can occur in adults and children. It can occur even in newborns and babies in the womb. A stroke can damage the brain and central nervous system. A stroke is serious and requires immediate medical attention.
“People who have received the J&J vaccine who develop severe headache, abdominal pain, leg pain, or shortness of breath within three weeks after vaccination should contact their health care provider. Health care providers are asked to report adverse events to the Vaccine Adverse Event Reporting System,” the statement said.
“Right now, I’d like to stress these events appear to be extremely rare,” Woodcock said. “However, COVID-19 vaccine safety is a top priority for the federal government, and we take all reports of adverse events following vaccination, very seriously.”
Dr. Anne Schuchat, the CDC’s principal deputy director, cautioned the blood clot symptoms should be differentiated from the more common flu-like symptoms present after vaccinations.
“While these events are very rare, we’re recommending a pause in the use of the J&J COVID-19 vaccine in order to prepare the healthcare system to recognize and treat patients appropriately, and to report severe events they may be seeing in people who receive the J&J vaccine,” Schuchat said.
“We are committed to an expeditious review of the available information, and to an aggressive outreach to clinicians so they know how to diagnose and treat” these reactions, Schuchat said.
“The safety and well-being of the people who use our products is our number one priority. We share all adverse event reports about individuals receiving our COVID-19 vaccine, along with our assessment of these reports, with health authorities in compliance with regulatory standards,” said Johnson & Johnson. “We are aware that thromboembolic events including those with thrombocytopenia have been reported with COVID-19 vaccines. At present, no clear causal relationship has been established between these rare events and the Janssen COVID-19 vaccine. We continue to work closely with experts and regulators to assess the data and support the open communication of this information to healthcare professionals and the public.”
It was only last Friday that Europe’s drug regulator European Medicines Agency (EMA) said that it was looking into Johnson & Johnson’s shot over blood clots, according to Reuters.
In total, four serious cases of rare blood clots with low platelets — one of which was fatal — have been reported in Europe after inoculation with Johnson & Johnson’s vaccine from its Janssen unit, the European Medicines Agency said.
“This announcement will not have a significant impact on our vaccination plan,” White House COVID-19 response coordinator Jeff Zients said in a statement. “Johnson & Johnson vaccine makes up less than 5 percent of the recorded shots in arms in the United States to date. Based on actions taken by the President earlier this year, the United States has secured enough Pfizer and Moderna doses for 300 million Americans,” he stressed.
For months, former New York Times reporter and author Alex Berenson has been warning about the possible side effects from the vaccines. Berenson reported on Twitter Tuesday that the Johnson and Johnson vaccine is almost identical to the Astra Zeneca vaccine being used in Europe, and that vaccine has seen larger numbers of the deadly clotting in women.
To be clear on what the numbers really are here:
Well over 100 women have become severely ill or died from an incredibly rare clotting condition after receiving the DNA/AAV #Covid vaccines . The known cases now are mostly @astrazeneca, but the @jnj vaccine is almost identical…
— Alex Berenson (@AlexBerenson) April 13, 2021
The denominator is in the range 40-50 million doses – 20 million in Britain, 20+ million in the EU. A dose is NOT a completed vaccination, as the @astrazeneca vaccine requires two doses.
So the real ratio here is far higher than 1 per million vaccinations…
— Alex Berenson (@AlexBerenson) April 13, 2021
Former President Trump meanwhile issued a statement Tuesday blasting the CDC for sidelining the Johnson & Johnson vaccine and suggesting that Pfizer was behind the push to have the J&J vaccine yanked off the market.
The Biden Administration did a terrible disservice to people throughout the world by allowing the FDA and CDC to call a “pause” in the use of the Johnson & Johnson COVID-19 vaccine. The results of this vaccine have been extraordinary but now it’s reputation will be permanently challenged. The people who have already taken the vaccine will be up in arms, and perhaps all of this was done for politics or perhaps it’s the FDA’s love for Pfizer.
The FDA, especially with long time bureaucrats within, has to be controlled. They should not be able to do such damage for possibly political reasons, or maybe because their friends at Pfizer have suggested it. They’ll do things like this to make themselves look important.
Remember, it was the FDA working with Pfizer, who announced the vaccine approval two days after the 2020 Presidential Election. They didn’t like me very much because I pushed them extremely hard. But if I didn’t, you wouldn’t have a vaccine for 3-5 years, or maybe not at all. It takes them years to act! Do your testing, clean up the record, and get the Johnson & Johnson vaccine back online quickly. The only way we defeat the China Virus is with our great vaccines!
– – –
Debra Heine reports for American Greatness.





I was diagnosed with rheumatoid arthritis about 10 years ago. 5 years ago, I started taking a Pfizer drug called Xel Janz (which was approved by the FDA in 2012 with NO warnings of side effects concerning heart problems or cancer).
Back on February of this year, I was taken to the emergency room and had to undergo open heart surgery where they replaced a valve, gave me a 5-way bypass and removed a clot from my heart. I have NEVER had ANY issues with my heart and have been very healthy and there is NO history of heart issues in my family. I was NOT overweight, did NOT eat poorly, etc. so I was NOT a “likely” candidate to have heart issues.
On Tuesday, April 6th, I was at my rheumatologist’s office for the first time since my surgery. I told him of my heart surgery. He proceeded to tell me that JUST LAST MONTH that Pfizer sent out a report stating that my medication was now found to cause heart issues including blood clots in the heart and lungs, heart attacks and even cancer. (After additional research, my rheumatologist was wrong. Pfizer knew about this and published their report in 2019.) SEVEN YEARS AFTER FDA APPROVAL!!!
So, can ANYONE tell me that after LESS than ONE YEAR of testing, ANY of these Covid vaccines are really safe?
Here is a good analysis of this: https://www.bitchute.com/video/THkikEAK2yc9/