COVID-19 vaccine supporters are fond of sneering at public figures who have called for the Food and Drug Administration to pull or at least re-evaluate the safety of the increasingly unpopular therapeutics, such as Health and Human Services secretary nominee Robert F. Kennedy Jr., cardiologist Peter McCullough and Florida Surgeon General Joseph Ladapo.
Read the full storyTag: Food and Drug Administration
Healthshare Org Urges Incoming Trump Admin to Make ‘Radical’ Change to FDA, Healthcare System
Solidarity HealthShare is calling on the incoming Trump Administration to fix the “nation’s broken healthcare system” with widespread changes to the Food and Drug Administration (FDA).
Read the full storyLawmakers Demand Investigation into U.S. Drug Companies Who Reportedly Worked with Chinese Military
A group of bipartisan lawmakers are demanding an investigation into U.S. drug companies with reportedly troubling ties to China, according to a letter obtained by the Daily Caller News Foundation.
The lawmakers raised alarm to the Food and Drug Administration (FDA) in a Monday letter that they had identified some U.S. pharmaceutical companies as having worked with the Chinese military, raising concerns that U.S. intellectual property is being siphoned off by Beijing. The lawmakers also pointed out that several U.S. pharmaceutical companies also conducted clinical trials in the Xinjiang province of China, a region known for “genocide” against religious minorities, according to a letter sent by the lawmakers to the agency.
Read the full storySCOTUS Refused to Ban Federal Censorship Pressure; It Could Make Churches Complicit in Abortion
When the Supreme Court reversed a preliminary injunction against several federal agencies and officials in June for “coerc[ing] or significantly encourag[ing]” tech platforms to suppress content, Washington state saw a new way to protect its mandatory abortion coverage in maternity healthcare plans from religious freedom challenges.
Five years into a lawsuit by Cedar Park Assembly of God against SB 6219, which includes criminal penalties up to prison, the Evergreen State argues that insurers won’t necessarily offer abortion-free plans if the court permanently bars it from enforcing surgical- and chemical-abortion coverage against such religious ministries that are opposed to abortion.
Read the full storyFDA Knew ‘Gender Affirming’ Puberty Blockers Increase ‘Suicidality’ in 2017, Promotes Them Today
Five months before the Food and Drug Administration issued a health warning on puberty blockers widely used off-label to treat minors with gender confusion, undermining a Department of Health and Human Services office that claimed “early gender affirming care is crucial to overall health and well-being,” an FDA leader acknowledged other health concerns.
Pediatric patients exposed to “gonadotropin-releasing hormone agonists,” most with central precocious puberty (CPP) and “a handful … transgender kids using the drugs off-label,” had an “increased risk of depression and suicidality, as well as increased seizure risk,” Division of General Endocrinology clinical team leader Shannon Sullivan told colleagues.
Read the full storyLouisiana Abortion Pill Reclassification Bill Heads to Governor’s Desk
The Louisiana state Senate approved a bill on Thursday that would place two abortion pills on the state’s list of controlled dangerous substances, sending the legislation to the governor’s desk for his signature.
The state’s House of Representatives passed the bill on Tuesday, which could make possession of the drugs a crime punishable by jail time or a fine. Surgical and medical abortions are already illegal in the southern state except in extreme cases, meaning it is already difficult to obtain the drugs legally. But now the possession itself without a prescription could get an individual up to five years in prison.
Read the full storyFDA Threatens Endangered Species with Shoddy Abortion-Drug Reviews: Lance Armstrong Investigator
Federal public health officials created strange bedfellows among animal-welfare advocates, scientists and vaccine skeptics for allegedly cutting corners in viral and COVID-19 vaccine research and oversight, possibly engineering a pathogen, then a cure that’s worse for some.
The Food and Drug Administration may be creating another odd couple in a case at the Supreme Court: environmental and pro-life activists.
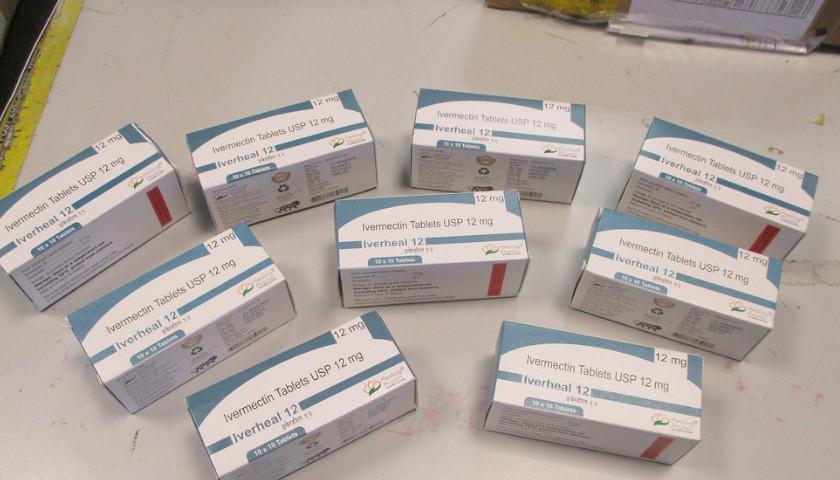
Read the full storyFeds Conceal Details About Anti-Ivermectin Campaign in Response to Doctors’ Reinstated Lawsuit
The Food and Drug Administration wants to continue its selective promotion of off-label drug use: good for COVID-19 vaccines, bad for alternatives to those vaccines. It just doesn’t want the public to see its full reasoning for the latter.
The FDA and the Department of Health and Human Services filed a renewed motion to dismiss a lawsuit by doctors claiming the agencies have a practice of demonizing ivermectin by conflating its human and animal doses and using “command” language, such as “stop it,” to discourage using the anti-parasite drug against COVID.
Read the full storyPeople in Florida Will Soon Be Able to Buy Drugs from Canada
The Food and Drug Administration (FDA) approved Florida’s request to import cheap prescription drugs from Canada on Friday.
Policymakers across the political spectrum have long sought to import drugs from Canada, where drug prices are lower, and Florida’s authorization makes it the first state to import drugs in bulk from America’s northern neighbor. Florida estimates that it may save as much as $150 million on drugs treating things like diabetes, hepatitis C and certain psychiatric conditions.
Read the full storyFDA Inspections of Foreign Drug Facilities Plummeted Since Before COVID-19, Study Shows
The Food and Drug Administration (FDA) has inspected fewer pharmaceutical manufacturing facilities compared to before the COVID-19 pandemic, with foreign facilities, including those from China, seeing the largest decreases, according to a study released in December.
In 2022, the total number of inspections of drug manufacturing establishments by the FDA decreased by 79% for foreign and 35% for domestic facilities compared to 2019, before the COVID-19 pandemic, according to a study by Emily Cuddy, Yun Peng Lu and David B. Ridley using data acquired through Freedom of Information Act requests. Despite the drop in inspections, there was no corresponding decrease in imports or manufacturing, while resources allocated by the FDA toward inspections surged per inspection.
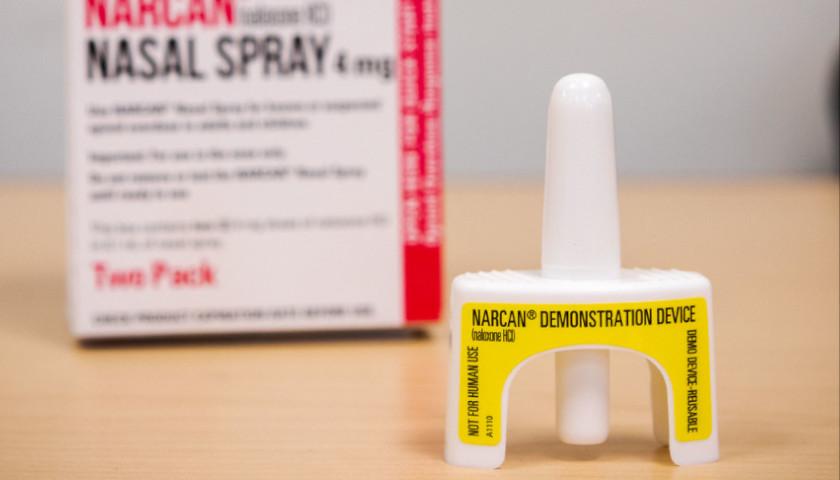
Read the full storyMore Restaurants, Bars Stock Up on Fentanyl, Opioid Overdose Reversal Drug as Deaths Soar
An increasing number of restaurants and bars across the country are keeping a stock of Naloxone, an antidote to fentanyl and opioid overdoses, according to The New York Times.
Local officials and nonprofit organizations are ramping up efforts to more bars and restaurants as overdoses become all too common in public spaces, according to the NYT. Between February 2022 and February 2023, there were more than 105,000 reported drug overdoses in the U.S., according to provisional data from the Centers for Disease Control and Prevention (CDC).
Read the full storyAppeals Court Says FDA Denunciations of Ivermectin Look Like ‘Command,’ not Advice
The Food and Drug Administration (FDA) is claiming in federal court that it never told doctors not to prescribe ivermectin to treat COVID-19. Federal judges aren’t buying it, and state medical boards that rely heavily on FDA guidance continue to investigate doctors for such prescriptions.
Echoing a federal district judge nine months ago, a three-judge panel of the 5th U.S. Circuit Court of Appeals pressed a Justice Department lawyer to reconcile the FDA’s repeated public denunciations of ivermectin as an off-label COVID treatment with its insistence that the agency is not liable for resulting investigations of doctors who prescribe or promote it.
Read the full storyMinneapolis Democrat Mayor Orders Police to Scale Back Arrests for Psychedelic Plants
Minneapolis Democrat Mayor Jacob Frey has ordered the city police department to stop enforcing most laws against using hallucinogenic plants.
Frey in announcing the order Friday pointed to the potential benefits of taking hallucinogenic plants to treat mental illnesses such as depression and post-traumatic stress disorder.
Read the full storyWorld Health Organization Labels Aspartame as a Possible Cancer Cause, FDA Disagrees
A World Health Organization (WHO) committee has released a report that finds the well known sweetener aspartame is a possible cause of cancer.
The new classification is based on a review of “limited evidence.” The U.S. Food and Drug Administration (FDA), however, disagrees with the report released Thursday, according to NPR.
Read the full storyCommentary: The FDA Must Partner with State AGs to Crack Down on Illegal Vapes and Keep Kids Safe
Millions of kids and teens in America are falling victim to an insidious campaign to get them hooked on illegal, disposable vapes that are made in China and intentionally marketed in youth-enticing flavors.
After years of inaction, the Food and Drug Administration (FDA) has finally said it will make compliance and enforcement against these products a priority. FDA’s decision to start taking action to stop the rising tide of illegal disposable vapes in youth-enticing flavors that are flooding our country from China is an important step forward but letters won’t be enough to get these products off our shelves.
Read the full storyPennsylvania State Senator Introduces Ban on Kratom Sales to Minors
A Pennsylvania state legislator is spearheading a bill to more stringently regulate the sale of the painkiller kratom.
The Kratom Consumer Protection Act, sponsored by state Senator Tracy Pennycuick (R-Red Hill), would ban the substance’s purveyance to anyone aged 21 or younger. The legislation would also limit the product’s potency, bar its combination with controlled chemicals and require its display of “adequate labeling directions for… safe and effective use….”
Read the full storySupreme Court Maintains Broad Access to Abortion Pill, Pending Litigation
The Supreme Court on Friday opted to preserve access to mifepristone while a challenge to the Food and Drug Administration’s approval of the drug makes its way through the courts. The Biden administration and mifepristone manufacturer Danco Laboratories had appealed to the court for relief. The court did not decide on the merits of the case, which will continue through the court system, the Associated Press reported.
Read the full storyCommentary: After Decades of Outsourcing to China, the U.S. is Running Out of Children’s Antibiotics
Acute shortages of orally delivered amoxicillin, penicillin and other children’s antibiotics throughout the 2022 and 2023 cold and flu season have made it difficult for doctors to treat normal childhood illnesses like ear infections, bronchitis, strep throat and rarer cases of infections caused after suffering Respiratory Syncytial Virus (RSV), and also sickle cell disease—for months.
The Food and Drug Administration (FDA) issued a warning about the amoxicillin shortage in Oct. 2022 just at the start of the cold and flu season. But since then, no statement has been issued by President Joe Biden about what appears to be an underreported public health crisis.
Read the full storyDespite Court Ruling, Arizonans Keep Access to Abortion Drug
Attorney General Kris Mayes said that a common abortion pill is still accessible in Arizona after two federal court rulings last week sparked confusion.
A Texas-based judge ruled that the Food and Drug Administration scrap its approval of mifepristone.
Read the full storyCleveland Proposes Ban on Sale of Flavored Tobacco Products
Mayor Justin Bibb proposes banning the sale in Cleveland of flavored tobacco products, including menthol cigarettes and flavored vape products.
The Columbus City Council approved a similar law in December of last year, and it will go into effect on January 1, 2024. Also in December, a bill to ban local governments from outlawing the sale of flavored tobacco products was approved by both chambers of the Ohio General Assembly; however, Governor Mike DeWine vetoed the measure.
Read the full storyOhio Congressman Calls Out FDA for ‘Illegal’ Approval of Mail-Order Abortifacients
U.S. Representative Bob Latta (R-OH-5) is leading a charge by federal lawmakers against the Food and Drug Administration’s (FDA) relaxation of safety requirements for abortion drugs so consumers can access them by mail.
The Bowling Green-area lawmaker coauthored a letter with U.S. Senator Cindy Hyde-Smith (R-MS) and garnered signatures from 75 other members of Congress to insist that the FDA’s recent actions violate federal law. In particular, the legislators object to the agency’s approval of chemical abortion-inducing substances while no longer requiring in-person dispensing.
Read the full storySusan B. Anthony List Applauds 22 Pro-Life Attorneys General, Including Tennessee’s Skrmetti, in Urging the FDA to Reverse New Policy on Abortion Drug
Susan B. Anthony Pro-Life America recently thanked a coalition of 22 attorneys general, including Tennessee Attorney General Jonathan Skrmetti, for sending a letter to the Food and Drug Administration (FDA) on the agency’s “illegal and dangerous” policy on mifepristone, a chemical abortion drug.
Read the full storyTop Epidemiologist Wants Pandemic Emergency Powers Ended, Insurer Death Data Released
One of the nation’s leading epidemiologists is declaring there is no basis for President Joe Biden to extend his emergency pandemic powers and that it is essential for insurers to release data showing deaths and injuries to those who have received COVID-19 vaccines.
Dr. Harvey Risch, professor emeritus at the Yale University School of Public Health, told Just the News on Friday evening that federal agencies have epically mishandled the pandemic strategy by substituting theories and politics for science.
Read the full storyGrowing Body of Evidence Disputes Claims That Puberty Blockers Are Safe, Reversible
Puberty blockers are widely touted by doctors and transgender activists as a safe and fully reversible way to pause puberty for children with gender identity issues, but a growing body of evidence is challenging those claims, according to The New York Times.
The drug prevents the surge in bone density that would normally occur during puberty, and patients can see lifelong bone issues that are never resolved, according to the Monday NYT article. Medical professionals are also challenging claims that the drug is reversible, arguing instead that blocking puberty permanently cements a child’s transgender identity and puts them on a path to lifelong biomedical intervention.
Read the full storyUS Senator Ron Johnson Presses Feds for Source of Vaccine at Military Bases after Whistleblower Allegations
Sen. Ron Johnson (R-Wisc.) is pressing the Pentagon, Food and Drug Administration, and the Centers for Disease Control and Prevention for answers after multiple whistleblowers raised concerns about the provenance of a Comirnaty-labeled COVID-19 vaccine shipped to military bases.
On Monday, nine military officers from across all the branches sent a whistleblower report to Congress regarding a COVID vaccine appearing at Coast Guard medical clinics. Key GOP senator presses feds for source of vaccine at military bases after whistleblower allegations
Read the full storyMiyares Joins Amicus Brief Supporting Decision to Vacate Travel Mask Mandate
Attorney General Jason Miyares joined an amicus brief opposing the Biden administration’s ongoing lawsuit over the CDC’s mask mandate for interstate travel. A district court vacated the requirement, but the CDC appealed, and the Health Freedom Defense Fund v. Biden case is now in the U.S. Court of Appeals for the 11th Circuit.
“Mask Mandates across the country have been lifted in virtually every aspect of daily life. For months, Americans have been traveling safely while making their own, autonomous decisions. The CDC mask mandate on public transportation, like air travel, is obsolete and no longer necessary – not to mention a clear example of federal overreach,” Miyares said in a press release.
Read the full storyCommentary: The Feds Pile Up Vaccine ‘Adverse Event’ Reports as They Decry Scaremongering Elsewhere
Since the Food and Drug Administration authorized the first vaccines for COVID-19 in late 2020, the government and much of the media have insisted that the medicines developed in record time are safe and effective. Those who raised questions about them have been routinely dismissed as conspiracy theorists.
Read the full storyFDA Vaccine Panel Recommends COVID Shots for Babies and Toddlers
The Food and Drug Administration’s (FDA) vaccine advisory panel unanimously voted Wednesday to recommend the Moderna and Pfizer vaccines for infants and young children despite an abundance of calls from physicians, children’s health organizations, and members of Congress to refrain from approving the shots for a population that shows the least risk for serious disease from COVID.
The Vaccines and Related Biological Products Advisory Committee (VRBPAC) voted, 21-0, in favor of recommending Emergency Use Authorization (EUA) for the Moderna and Pfizer mRNA COVID vaccines for infants, toddlers, and preschool-age children.
Read the full storyDoctor Crashes FDA Meeting and Shares the Whistleblower Story They Ignored
A doctor “crashed” a Food and Drug Administration’s meeting with outside vaccine experts earlier this week, to share a whistleblower’s story about the data integrity issues that plagued one of Pfizer’s clinical trials.
In September of 2020, a researcher from an organization testing Pfizer’s vaccine at several sites in Texas, emailed a complaint to the FDA, informing the agency of the company’s dangerously shoddy research practices. The FDA took no action on her email, and Pfizer continues to use the company.
Read the full storyPfizer CEO Calls for Another Booster Shot for All Americans
On Sunday, the chief executive officer of Pfizer said that Americans should be prepared to receive a second booster shot of the Coronavirus vaccine, which would mark the fourth overall shot that has been forced on the American public.
As reported by Politico, Albert Bourla made his remarks in an interview with CBS’ Margaret Brennan, where he said that his company was preparing to submit “a significant package of data about the need for a fourth dose” to the Food and Drug Administration (FDA).
Read the full storyAbortion Pills Now More Common Than Surgical Abortions
Medication-induced abortions accounted for 54% of all abortions in the U.S. in 2020, according to the Guttmacher Institute.
Abortion pills have grown in popularity since they were first introduced in 2000, the Guttmacher Institute reported. And rules requiring women to receive their first two abortion pills at a clinic or doctor’s office were lifted during the pandemic, allowing women to speak with doctors via “telemedicine” and get the pills by mail, The New York Times reported.
Read the full storyFDA Executive Says on Hidden Camera That Yearly COVID Shots Will Be Mandatory for All Americans – Including Toddlers
The Biden administration plans to make yearly COVID shots mandatory for all Americans, including young children, a Food and Drug Administration executive told a Project Veritas undercover journalist on hidden camera.
In the sting video released on Tuesday, Christopher Cole, Executive Officer of Countermeasures Initiative for the FDA, said: “Biden wants to inoculate as many people as possible.” According to Project Veritas president James O’Keefe, Cole has “over 20 years experience” at the FDA, and “claims to be directly involved in the approval process.”
Read the full storyFDA Announces Postponement of Approval of COVID Vaccine for Babies and Young Children
Pfizer and the Food and Drug Administration (FDA) said Friday they are delaying their plan for Pfizer’s Emergency Use Authorization (EUA) for its coronavirus vaccine for children under five years old due to insufficient data on the efficacy of a third dose.
Pfizer announced February 1 FDA had asked the drug company, and its partner BioNTech, to submit data on a COVID vaccine series for babies as young as six months old and young children up until age five.
Read the full storyPfizer Plan for COVID Vaccine Series for Babies of 6 Months Draws Fierce Controversy
Pfizer announced last week the Food and Drug Administration (FDA) had asked the drug company, and its partner BioNTech, to submit data on a COVID vaccine series for babies as young as 6 months old.
Albert Bourla, chairman and CEO of Pfizer, said in the statement:
As hospitalizations of children under 5 due to COVID-19 have soared, our mutual goal with the FDA is to prepare for future variant surges and provide parents with an option to help protect their children from this virus. Ultimately, we believe that three doses of the vaccine will be needed for children 6 months through 4 years of age to achieve high levels of protection against current and potential future variants. If two doses are authorized, parents will have the opportunity to begin a COVID-19 vaccination series for their children while awaiting potential authorization of a third dose.
Read the full storyGovernor Ron DeSantis Shreds Biden over Decision to Revoke Emergency Use Authorization for Monoclonal Antibody Treatments
Florida Governor Ron DeSantis shredded President Joe Biden’s administration over the decision to revoke the emergency use authorization for Regeneron and Eli Lilly monoclonal antibody treatments.
According to the Food and Drug Administration, the treatments are not effective against the Omicron variant. Because the variant accounts for most cases of the coronavirus across the country, leaders of the agency limited its use.
Read the full storyCommentary: What Reverend Martin Luther King, Jr. Would Say About Biden’s New COVID-19 Policy
Given the Biden administration’s recent effort to prioritize COVID-19 treatments based on race, it is more important than ever that we remember – and practice – the teachings of Reverend Martin Luther King Jr.
Last week, the Food and Drug Administration released new guidance to medical professionals which listed “race or ethnicity” as high risk factors for doctors to consider when prescribing a new monoclonal antibody known as Sotrovimab. Other high-risk factors included obesity, pregnancy, and other health conditions which would make a person less able to fight the virus. The new guidance means a person’s race could qualify him or her for treatment ahead of others who need the drugs.
Biden administration officials have cited high rates of diabetes and other health issues which are prevalent in non-white and non-Hispanic communities as reasons to include the new criteria. Officials in New York and Minnesota have also prioritized treating non-white patients, but they have more overtly cited historic health care disparities as a justification.
Read the full storyVersion of Pfizer Vaccine that Predates FDA Approval Still Being Distributed in Pennsylvania
Of the three companies producing COVID vaccines in the U.S., only one—Pfizer Inc.—has yet gained full FDA approval, and at least some Pfizer vaccines currently being administered in Pennsylvania come from inventory that predates that approval.
On Aug. 23, the U.S. Food and Drug Administration (FDA) approved a Pfizer shot to prevent severe COVID-19 cases. Like Johnson & Johnson and Moderna, Pfizer had been a manufacturing vaccine to fight the coronavirus under federal emergency-use authorization (EUA). The injection produced by Pfizer under EUA is known as Pfizer BioNTech and the company’s post-FDA approval vaccine is called Comirnaty (pronounced kuh-MUR-nit-ee).
Read the full storyFDA Claims It Needs 55 Years Before Revealing Data on Approval of Pfizer Vaccine
On Monday, the Food and Drug Administration (FDA) requested that the courts allow the agency to wait until the year 2076 to release all of the relevant documents regarding the approval of the vaccine developed by Pfizer and BioNTech, as reported by the Daily Caller.
The FDA made its request after a lawsuit was filed against the agency by the group Public Health Medical Professionals for Transparency (PHMPT). The PHMPT had previously made a Freedom of Information Act (FOIA) request on September 9th asking for the release of the vaccine approval documents; after the FDA denied the request, the group filed its lawsuit on September 16th.
The FDA concluded that there were roughly 329,000 pages in total that would qualify under this FOIA request. In its appeal to the courts, the agency said that, at most, employees would be able to “process and produce the non-exempt portions of responsive records at a rate of 500 pages per month.” Under this process, the FDA said that it would hand over prioritized documents to the plaintiff, and release non-exempt documents on a “rolling basis.”
Read the full storyBiden Announces Former Obama FDA Commissioner Robert Califf to Lead FDA Again
President Joe Biden on Friday announced the nomination of Robert Califf to head up the Food and Drug Administration again, urging the Senate to quickly confirm him a second time.
Califf, who served in the same role near the end of then-President Barack Obama’s second term, is “one of the most experienced clinical trialists in the country, and has the experience and expertise to lead the Food and Drug Administration during a critical time in our nation’s fight to put an end to the coronavirus pandemic,” Biden said in a statement announcing the pick.
“I am confident Dr. Califf will ensure that the FDA continues its science and data driven decision-making,” the president added, pointing out that Califf enjoyed “strong bipartisan support in the Senate in 2016.”
Read the full storyProposed Law Would End Ohio Sales Tax on Guns, Ammunition, Knives
Sales tax would no longer be collected on guns, ammunition and knives in Ohio if a bill planned for introduction in the state House of Representatives becomes law.
State GOP Rep. Al Cutrona recently announced he will introduce legislation that would exempt those items from sales tax, saying the move would help make gun, ammunition and knife retailers and manufacturers more competitive with neighboring states.
Read the full storyBrnovich Requests Restraining Order Against Biden Vaccine Mandate
Arizona Attorney General Mark Brnovich asked the U.S. District Court in Arizona for a temporary restraining order and nationwide preliminary injunction against the Biden Administration’s COVID-19 vaccine mandates.
“The COVID-19 vaccine mandate is one of the greatest infringements upon individual liberty, federalism, and the separation of powers by any administration in our country’s history,” Brnovich said in a news release Friday.
Read the full storyCommentary: Defense Department Pulls a Bait and Switch on Vaccines
On August 24, Secretary of Defense Lloyd Austin issued a memo to senior Pentagon leadership announcing that he was implementing a mandatory COVID-19 vaccination policy for all military service members. The day before, the FDA had issued full authorization to Pfizer for their Comirnaty COVID-19 vaccine product (the nomenclature of which is meant to be a mashup of the words “COVID”, “mRNA”, and “community”) . At first glance it would seem that the mandatory vaccination policy, while scientifically unsound and strategically foolish, was at least a policy being implemented according to both the letter of the directive and in accordance with the law. But a further examination of the facts and the manner in which this order is being implemented makes clear that the military’s implementation of this order is illegal and highly unethical.
In the memo, Secretary Austin issued a directive and a promise, that “Mandatory vaccination against COVID-19 will only use COVID-19 vaccines that receive full licensure from the Food and Drug Administration (FDA), in accordance with FDA-approved labeling and guidance.” The problem with this is that the Comirnaty vaccine product that was approved by the FDA is not available anywhere in the Military Health System. It is not even in production, according to the military’s TRICARE healthcare providers. If a soldier goes to a military hospital or a private provider to receive an approved Pfizer COVID vaccine, he will be administered the unapproved Pfizer-BioNTech vaccine which is a vaccine that is not approved but has been administered under an Emergency Use Authorization (EUA). We are told that this is but a brand name difference, that the formulation is the same, and they can be used interchangeably. But as the FDA was approving the Comirnaty product, they were renewing the authorization for the Pfizer-BioNTech product. If it’s just a matter of brand name, why issue an approval for one brand name and an EUA renewal for the other? This is because they are not actually the same.
Read the full storySidney Powell Sues Defense Department over Vaccine Mandate
Former Trump attorney Sidney Powell announced Wednesday that she is suing the Defense Department in regards to their vaccine mandate.
According to The Hill, Powell is representing the Texas-based group “Defending the Republic” in a lawsuit against Defense Secretary Lloyd Austin in regards to the military’s mandatory vaccination requirements.
Read the full storyMinnesota Lawsuit Forces Company to Accept Blood Donations from Transgender People
The recently announced outcome of a Minnesota lawsuit forces one of the nation’s largest plasma companies to accept blood from transgender donors who were previously considered too high risk.
CSL Plasma is one of three companies that are responsible for collecting over 75% of the total plasma donated in the U.S. For decades, the FDA has recommended caution when collecting blood and plasma from men who have sex with other men, as these individuals pose a higher than average risk of transmitting bloodborne diseases like AIDS. CSL apparently adhered to this guidance by not collecting plasma from biological men who identify as transgender women or nonbinary people, since these individuals are likely to engage in sexual relationships with other men.
Read the full storyLaw Professor Accuses University of Violating Federal Trade Commission Rules with Mask Mandate
A business law professor who has been put on paid leave for refusing to wear a mask in class is defending his actions with an unexpected authority: the Federal Trade Commission (FTC).
“[B]y requiring employees to wear a mask, you are promoting the idea that the mask can prevent or treat a disease, which is an illegal deceptive practice,” David Clements, who teaches consumer law at New Mexico State University (NMSU), told provost Carol Parker in a Sept. 13 letter.
Read the full storyTelehealth Abortions Are Available to Virginians
Planned Parenthood of Metropolitan Washington, D.C., (PPMW) is now providing telehealth abortions to people with addresses in Virginia, Maryland, and D.C., according to a September 10 press release. After a phone screening and an online consultation, PPMW mails abortion drugs to the patient. Total cost for the service is $525, including a follow-up consultation and pregnancy test.
Read the full storyFDA Says It Does Not Buy Fetal Tissue – Any More
The Food and Drug Administration assured the Daily Caller News Foundation Friday that it has not entered into any contracts “for the purchase of human fetal tissue” since 2018.
The agency’s response follows the release of documentation obtained by Judicial Watch showing that the FDA procured fetal organs, tissue, and heads for research that involved “humanized mice.” Previous documents uncovered by Judicial Watch found that the FDA requested “fresh and never frozen” fetal organs.
“I’ve been doing this for 23 years. These documents we’ve gotten from the FDA and our other lawsuit…they are the worst things I’ve ever seen,” Judicial Watch President Tom Fitton told the Daily Caller News Foundation Friday. “The most troubling documents I’ve ever seen.”
Read the full storyFDA Will Need More Time to Decide If Juul Can Sell E-Cigarettes
Health officials delayed a decision Thursday on whether e-cigarettes made by Juul and other top companies can stay on the U.S. market.
The Food and Drug Administration (FDA) said it needs longer than the Thursday deadline to determine if Juul and other select companies’ products can continue to be sold in the U.S., according to a press release.
Read the full storyReport: Top Health Officials Tell White House to Pause Vaccine Booster Plan
Top U.S. health officials told the White House pandemic coordinator on Thursday to scale back the Biden administration’s plan to administer the coronavirus booster shots to individuals in September, The New York Times reported.
Dr. Janet Woodcock, the acting commissioner of the Food and Drug Administration (FDA), and Dr. Rochelle P. Walensky, the director of the Centers for Disease Control and Prevention (CDC), told White House Coronavirus Response Coordinator Jeffrey D. Zients that they need more time to collect and analyze the necessary data relating to the booster shots, The New York Times reported.
The doctors told Zients that their agencies might be able to determine whether to recommend boosters for recipients of the Pfizer-BioNTech vaccine in the coming weeks, according to the Times.
The two doctors presented their argument to Zients at a meeting on Thursday. It is unclear how Zients responded to the news.
Read the full storyFDA Bans 55,000 Flavored E-Cigarette Products
The U.S. Food and Drug Administration banned 55,000 e-cigarette products on Thursday for their failure to prove they didn’t pose a threat to public health.
The FDA announced that thousands of products from three vape companies, JD Nova Group LLC, Great American Vapes, and Vapor Salon, didn’t prove the benefit to adult smokers negated the “well-documented, alarming levels of youth use of such products.”
Read the full story